Fashion >> Forward
For Daniel Mukasa, the future of wearables requires sweating the small stuff.

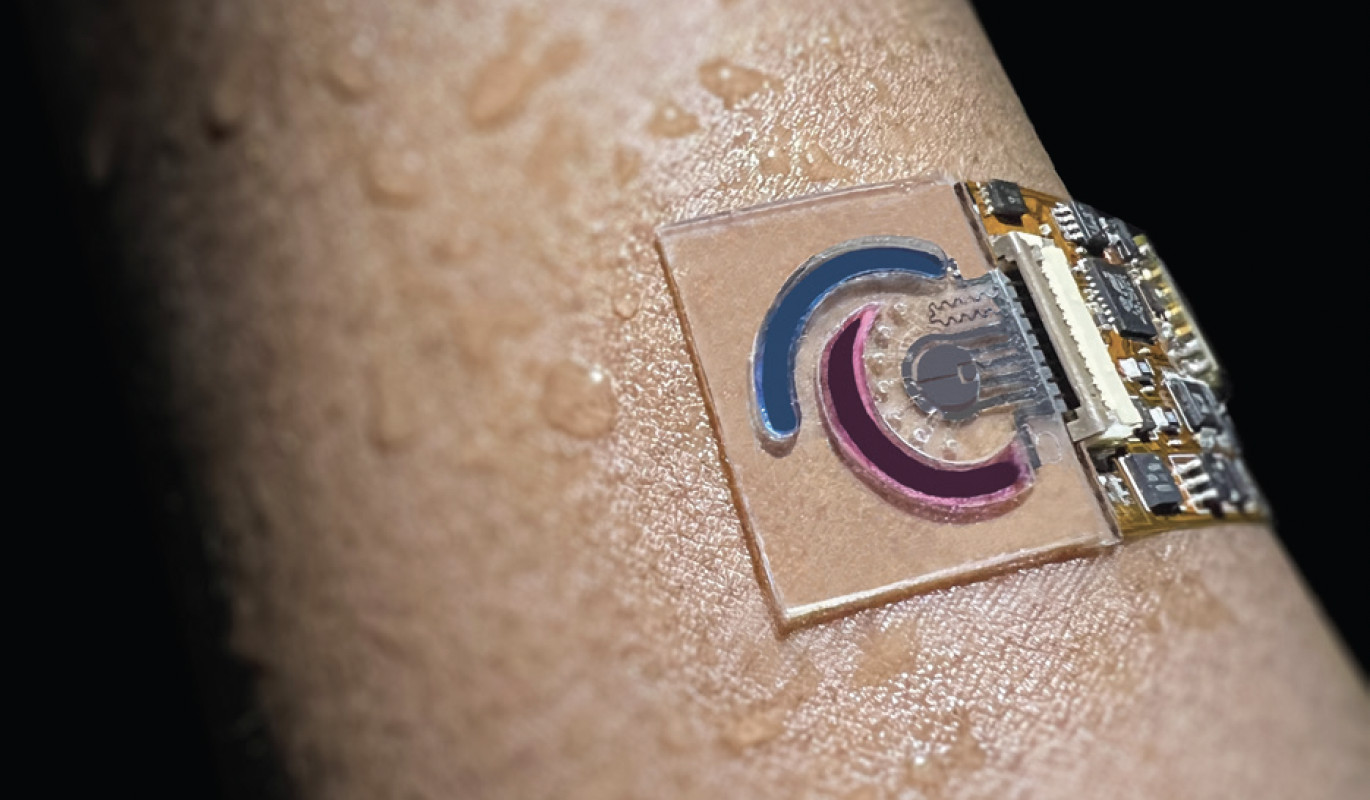
“My dad is an engineer and my mom is in health care,” Mukasa says, “So I kind of ended up in this middle field of biomedical engineering. I wanted to do engineering because I think with a very technical mind, but I wanted it to help people. Working on biomedical problems–where I could, say, diagnose a disease early on–felt really exciting.”
While completing his undergraduate studies at Oberlin College, Mukasa set his sights on Caltech. “I wanted to come back home, come back to the sun, and do amazing physics while also trying to help people’s lives,” he says. “And I landed in the perfect spot for that.” Specifically, he landed in the innovative lab of Wei Gao, assistant professor of medical engineering, who has been developing wearable sensors that monitor biomarkers in sweat to allow for the detection and management of various diseases and health conditions.
Thanks to the diffusion of molecules from the bloodstream to the sweat duct, explains Mukasa, “almost everything that you find in a blood profile you’ll also find in your sweat profile.” But unlike blood tests, whose results are a snapshot in time, sweat monitoring is non-invasive and offers continuous, real-time data. Each sensor is designed to detect a specific metabolite or nutrient, including amino acids, vitamins, hormones, and proteins.
For example, Mukasa has worked on one device, worn on the forearm, that tracks the amino acid phenylalanine for a rare but serious disease called phenylketonuria. Another monitors glucose levels, which can correlate to diabetes. There are also applications to monitor women’s reproductive cycles. “There’s a company, Oura, that [currently] makes a ring which tells a woman roughly where she is in her cycle, primarily from changes in body temperature—but this isn’t nearly as accurate as what you could get from a device that’s actually tracking the estrogen coming out of you.”
Mukasa says that some people in the lab find a niche, concentrating their efforts on a certain disease. “I’m really interested in all diseases,” says Mukasa, “so I’ve been focused on how to make the chemical sensor as accurate as possible for any biomarker—so that once we know something correlates to a disease, we can just make a device and ship it out immediately.”
While honing the sensitivity and accuracy of the sensors remains a difficult task, another challenge has already been significantly mitigated: the time it takes to find the correct materials and chemical procedures to construct each device. The process used to take years, but thanks to AI, which has brought considerable innovation and transformation to the entire field of materials science, the timeline is now often a matter of weeks. “We spent two to three years just detecting three biomarkers,” says Mukasa. “Now, with AI, if you’re able to train the right model, it scales things down by four to five orders of magnitude. We’re almost at the point where we can automate the entire development process, which is really exciting.”
Still many generations away, however, is the major goal of early cancer detection. “This isn’t necessarily an impossible goal,” says Mukasa, “because there have been reports of dogs being able to smell cancer on people—so there is some type of chemical profile change that happens. Maybe we can find the biomarkers for this. It would take more in-depth studies and years of research to find what exact chemical, or chemicals, are needed to make a diagnosis. It’s like needles in a haystack—but when they are found, it will open the doors to the potential of early diagnosis.”
As for his own future, Mukasa plans to pursue a career in academic sciences. “I want to open up my own research lab and continue making these devices and span out as much as I can into the health care field,” he says. “Drug discovery has been particularly interesting to me because you can’t just diagnose the diseases—at a certain point you have to treat them.”

Jeremy Arnold is a freelance writer and copyeditor whose work has appeared in Variety, The Hollywood Reporter, and Moviemaker. He has written four books on classic cinema, with his latest, a newly expanded edition of Christmas in the Movies, published last year. Jeremy appears periodically on Turner Classic Movies as a guest presenter and programmer, and has recorded audio commentaries for the Blu-ray release of many classic films.
Related Articles
-
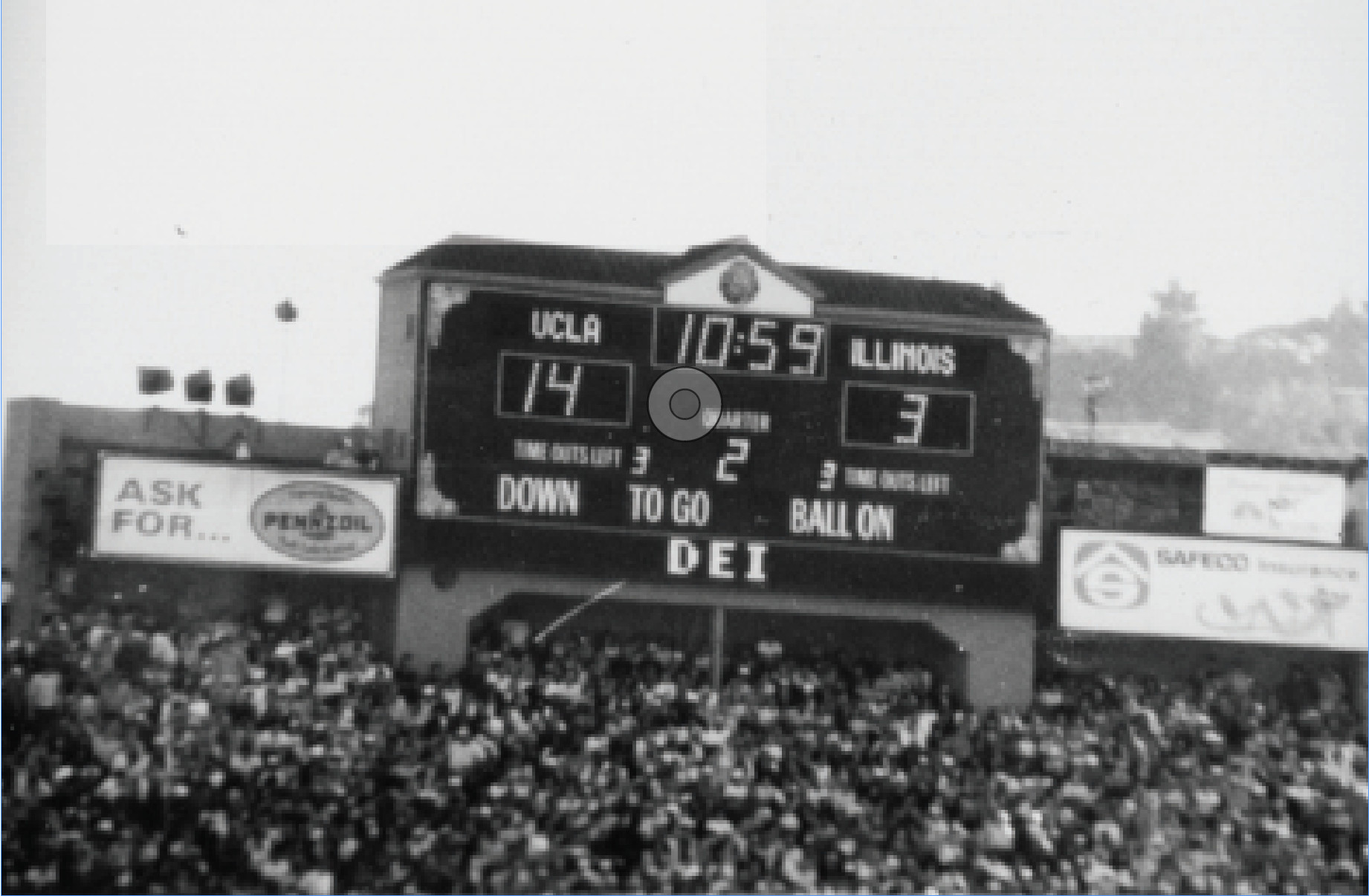
A Legendary Hack Turns 40
In this moment of Caltech mischief that required months of planning, students Ted Williams, PhD (BS ’84) and Dan Kegel (BS ’86) manipulated the sco...
-

The Pioneers
On the 50th anniversary of their graduation, the first four-year female graduates reflect on their time on campus—and where they went from here.
-

All In
The key to a more sustainable future? Make sure everyone can join the hunt for solutions, says National Energy Technology Laboratory director Maria...